A chloride dissolves appreciably in water. When placed on a Pt wire...
Published at : October 13, 2022
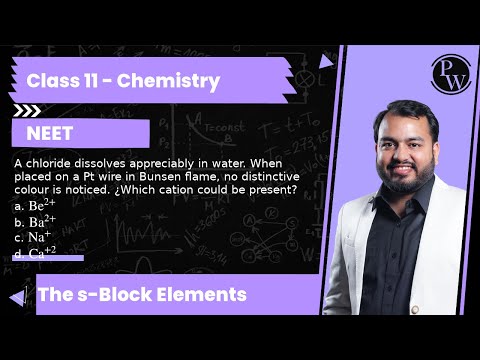
A chloride dissolves appreciably in water. When placed on a Pt wire in Bunsen flame, no distinctive colour is noticed. ¿Which cation could be present?
a. \( \mathrm{Be}^{2+} \)
b. \( \mathrm{Ba}^{2+} \)
c. \( \mathrm{Na}^{+} \)
d. \( \mathrm{Ca}^{+2} \)
📲PW App Link - https://bit.ly/YTAI_PWAP
🌐PW Website - https://www.pw.live
a. \( \mathrm{Be}^{2+} \)
b. \( \mathrm{Ba}^{2+} \)
c. \( \mathrm{Na}^{+} \)
d. \( \mathrm{Ca}^{+2} \)
📲PW App Link - https://bit.ly/YTAI_PWAP
🌐PW Website - https://www.pw.live

pw